Back to Basics
Hydrogen is one of the most common elements in the world — making up 75 percent of all visible matter and more than 90 percent of all the atoms in the universe. At the same time, hydrogen bonds are some of the weakest molecular bonds known to occur between atoms. As such, these bonds are difficult to observe, much less study.
As scientists endeavor to understand the number one element in the periodic table, the ability to see hydrogen bonds is something they’ve only dreamed of. But now it’s become a reality.
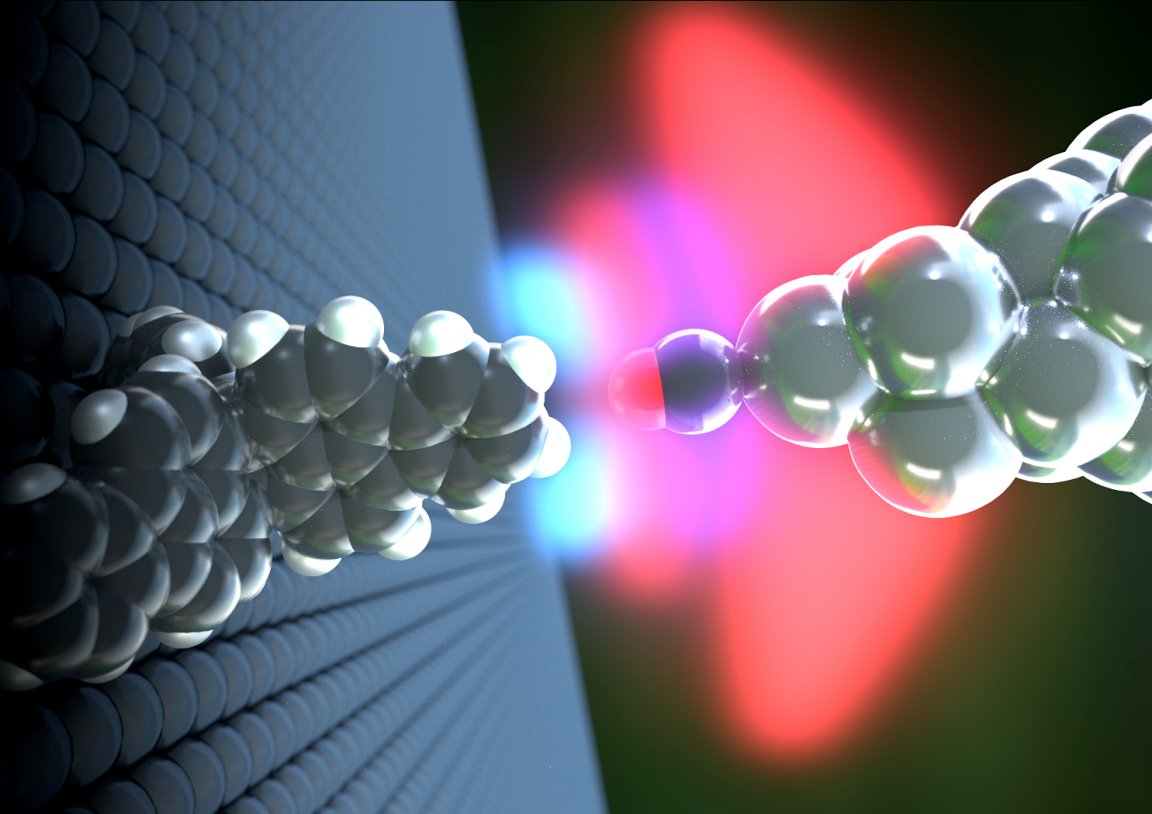
Researchers from the University of Basel’s Swiss Nanoscience Institute, in collaboration with colleagues from Japan, published a paper in the journal Science Advances detailing how they’ve managed to get a clear image of hydrogen bonds using what’s called an atomic force microscope (AFM) — which is a scanning probe microscope with very high resolution capable of visualizing and measuring minuscule forces, like those of a hydrogen.
“The hydrogen atom — the smallest and most abundant atom — is of utmost importance in physics and chemistry,” the researchers wrote in their paper’s abstract. “Although many analysis methods have been applied to its study, direct observation of hydrogen atoms in a single molecule remains largely unexplored.”

Using compounds known as propellanes — because, yes, they resemble propellers — the researchers were able to measure the force and distance between an oxygen atom and two hydrogen atoms (the components of water). These were observed using an AFM that was made extremely sensitive to hydrogen by adding a carbon monoxide layer at its tip. The carbon monoxide then formed a bond with the tip of the propellane compounds. It was this bond that the researchers studied and found similar to established hydrogen bond calculations.
Like You’ve Never Seen Them Before
Scientists haven’t been able to study hydrogen bonds because, aside from their weak nature rendering them rather vulnerable and easy to break, hydrogen atoms are also as small as atoms could get. Don’t underestimate these hydrogen bonds, however, as they play key roles in many of nature’s wonders. Hydrogen bonding plays an important part in giving water its properties, for example.

If you want to go up close and personal, hydrogen bonds keep the DNA’s double-helix structure together, becoming the building blocks to life’s building blocks. Therefore, this research could shed new light on our genetic structure. “Our … calculations confirm the signature of directional bonding, characteristic of very weak hydrogen bonding,” the researchers wrote in the study. “The direct measurement of the interaction with a hydrogen atom paves the way for the identification of three-dimensional molecules such as DNAs and polymers.”
Understanding the most basic of nature’s atomic bonds opens up new possibilities in understanding the physics of our world. “[H]ydrocarbons are one of the most varied and functionalized products at the heart of engineering, chemistry, and life, and hydrogen is often critical in their function,” the Swiss team added. This research could be the first step for bringing the exploration of the material world to the next level.