Precise Timing
The complexity of the human body and its physiology — features such as the immune system, for example — demand an astronomical level of precision in both timing and formation. Each genetic element must be activated at an exact moment and location to function properly. Cells mature to take over their intended functions in “enhancer” clusters, but in order to be expressed, these clusters must first reach specific “promoter” regions across long distances of genomic wasteland.
These two elements must be paired up in just the right way for the systems of the human body to function properly. In fact, discord between promoting regions and enhancer clusters can cause diseases such as lymphoma and leukemia. In other words, a lack of timing at the physiological level can be fatal. So, how does the body get this right?
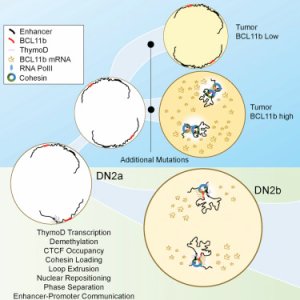
University of California San Diego biologists have discovered the linchpin to what they’re calling the “big bang” of immune cell development: the previously overlooked “non-coding” regions of DNA between genes.
They found that these regions of DNA activate a change in DNA’s 3D structure that unites promoters and enhancers with a high degree of accuracy. The process they observed in mice allowed T cells to develop properly. These cells are the building blocks of the immune system, which is why problems with their development can cause diseases such as leukemia and lymphoma.
Harnessing Forgotten DNA
This new research illustrates the extremely precise nature of our biology, a point not lost on UC San Diego Division of Biological Sciences laboratory head Cornelis Murre.
“Nature is so clever. We think of the genome as an unstructured strand, but in fact, what we are seeing is a highly structured and meaningful design,” he noted in a press release. “The process of architecture remodeling we’ve described allows the enhancer and promoter to find each other in 3D space at precisely the right time. The beauty is that it’s all very carefully orchestrated.

Murre and the rest of the research team believe that although their results were focused on T cells in mice, this same mechanism may be present throughout plant and animal species. “We have seen one example, but there are likely many others all occurring at the same time when cells are moving along the developmental pathway — that’s kind of amazing,” he asserted.
Understanding T cell development can also help us understand how and why the process fails, perhaps illuminating ways to prevent this failure and the diseases that result from it. “The implications of these results are not only how normal T cells develop, but that tumor suppression is regulated through this mechanism, at least in part,” said Murre. “Ultimately, we may be able to fix mutations associated with disease and these forgotten strands of DNA.”