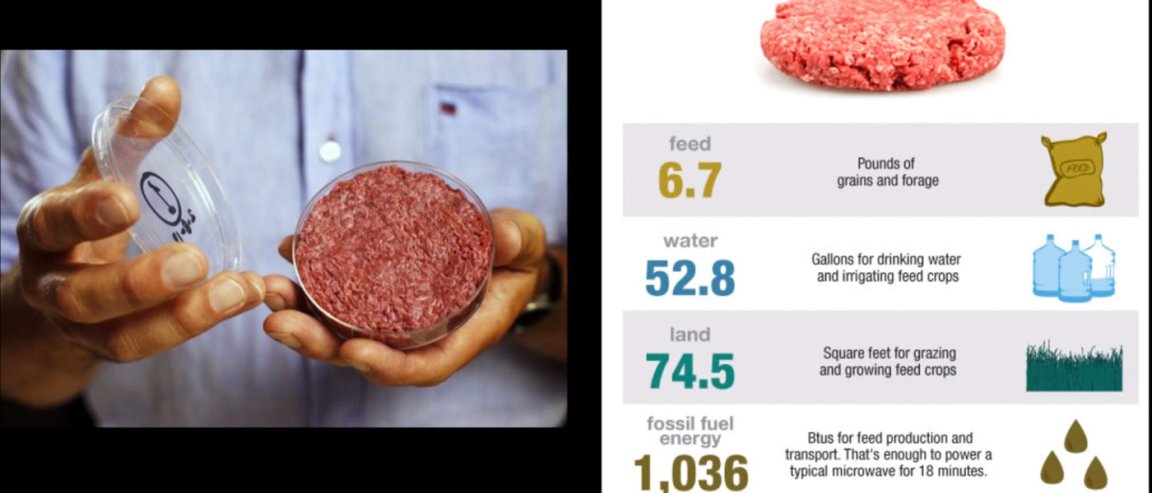
Meat production is one of the most resource-intensive areas in food production. As the human population grows, demand for meat continues to increase, as does its burden on the environment.
To make a quarter-pound hamburger, for instance, it takes 6.7 pounds of grain to feed the cattle, 52.8 gallons of water for drinking and irrigating feed crops, 74.5 sq. ft. of land for grazing and growing, and 1,036 btus of fossil fuel energy for feed production and transport.
It’s clear that a new feasible and sustainable strategy is needed.
Cultured, or lab-grown meat is one of the possible solutions to increasing the efficiency of meat production. In cultured meat, animal protein products are grown from isolated biopsies of animals. This sidesteps many of the ethical issues associated with conventional meat.

However, edible cultured meat has been extremely expensive to produce so far. Over time, cost will go down as techniques are refined, but other challenges remain, such as finding the most efficient growth medium and replicating the proper taste.
One of the challenges that seems to have the least obvious solution is recreating the texture of meat. Quinn Fucile might have the answer.
Fungal Mycelia
In the video Fixing Cultured Meat– Fungal ECM, Fucile explains that getting the proper texture for cultured meat requires replicating the extracellular matrix (ECM) as well as larger strands of connective tissue within the piece of meat.
Stem cells responsible for the ECM and connective tissue could be cultured alongside muscle and fat cells for this purpose, but getting them to behave and develop into the proper structure is another issue entirely.

Instead, Fucile proposes establishing a preliminary ECM scaffold for cultured meat production using genetically engineered fungus. There’s really only a superficial structural similarity between the ECM in animal tissue and the filaments of fungal mycelia.
To serve as an appropriate foundation for animal muscle tissue, the fungus will need some modifications. First, the production of certain animal ECM proteins and a programmed kill switch. Fucile believes both of these are feasible.
Modifying the Fungus
Getting the outer surface of the mycelia to chemically resemble animal ECM is the biggest challenge. The cell wall of the fungi is primarily made of a tough, semi-transparent substance called chitin, along with a small amount of protein. The key first step is down-regulating the production of chitin and increasing the percent of cell wall composed of protein.
There has already been successful genetic engineering of multicellular fungus using the latest genome editing tools. The next step will just be adding to or replacing the fungal structural proteins with animal collagen. Both collagen and fungal structural proteins are exported from the cell in a similar fashion. Once the challenge is met it is possible that the foreign proteins would be readily integrated into the cell wall.
The next modification needed is a programmable kill switch. Scientists have already developed a highly efficient and sensitive gene circuit that allows for programmed cell death with the addition of a normally harmless substance into the growth medium.
Once the modified fungus have grown sufficiently so that its mycelia provide a suitable scaffold for animal tissue, the kill switch will be initiated. This will prevent the fungus from draining any further nutrients from the growth medium and competing with the animal tissue. It will also leave behind the structural trends of the mycelia, ideally composed primarily of animal collagen.
From there, the scaffolding would be seeded with animal stem cells, including those associated with muscle fat connective tissue and perhaps even vasculature.
Mass Production
The final texture of the product would depend on how efficiently the fungus can be made to express collagen, as well as how much initial scaffolding is necessary to start the process. It may be that the fungus only needs to provide a very loose structure before animal stem cells can take hold.
This is, however, all a theory. Fucile is looking for “someone out there who can make it a reality.”
If Fucile’s idea works, the modified fungus could be used to grow these structures on mass and very efficiently. The mycelia naturally produce these types of structures and it may be possible to modify the growing conditions or genetics to get different structures and, therefore, final textures.
“Once established,” concludes Fucile’s video, “the fungus based scaffolding can then be sectioned off and used to grow a wide variety of cultured meat products, making highly efficient cultured meat a viable and appealing replacement for many everyday uses.”