
CRYSTALLINE WATER
Ice, whether in the form of a snowflake or in ice cubes, is basically a form of solid crystalline water. But scientists have previously found that the vast majority of the ice that we know is simply one form of this crystalline structure, identifying as many as 17 possible structures that ice could take.
Now there might be one more addition to that list. A University of Nebraska-Lincoln-led research team has predicted a new molecular form of ice that has yet to be discovered. Their study posted in the journal Science Advances shows that their proposed ice would be about 25 percent less dense than any other previous structure including a record-low form synthesized by a European team in 2014.
If they can synthesize this ice, it would be the 18th known crystalline form of water—and the first to be discovered in the United States since before World War II.
“We performed a lot of calculations (focused on) whether this is not just a low-density ice, but perhaps the lowest-density ice to date,” said Xiao Cheng Zeng who co-authored the study. “A lot of people are interested in predicting a new ice structure beyond the state of the art.”
This new discovery is simply the latest in a long line of ice-related research from Zeng who previously found two-dimensional “Nebraska Ice” that contracts instead of expands when frozen under certain conditions.
Zeng and his team used a computational algorithm and molecular simulation to determine the ranges of extreme pressure and temperature under which water would freeze into the predicted structure. That structure is in the form of a clathrate, a series of water molecules that form a cage-like structure.
SYNTHESIZING ICE
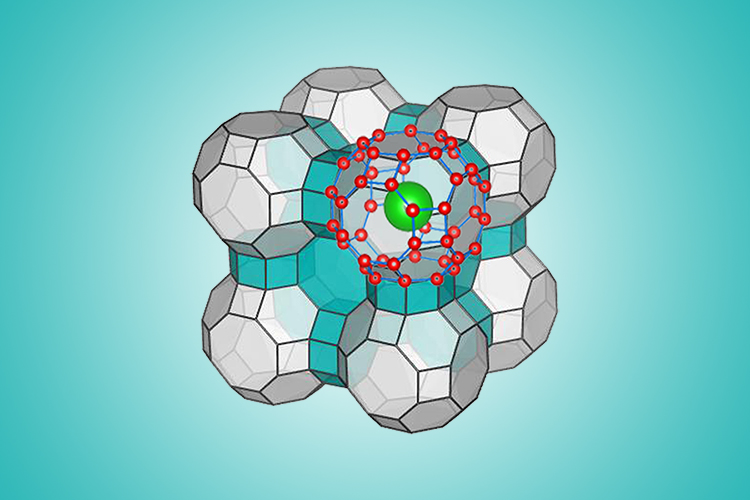
Scientists have previously held that this cage-like structure could only be structurally stable when it houses a “guest molecule” such as methane as seen naturally in clathrates found on the ocean floor and in permafrost. However, Zeng and his colleagues have figured out that their clathrate would remain stable despite the eviction the guest molecule.
Synthesizing the clathrate will require some effort. The team’s calculations estimate that this new ice will be formed only when water is placed inside an enclosed space which is subjected to ultra-high, outwardly expanding pressure. At around 250 Kelvins (-10 F), this space would have to be surrounded by expanding pressure about four times greater than what is found at the Pacific Ocean’s deepest trench. As it approaches absolute zero, the pressure needed would be equivalent to a person shouldering 300 jumbo jets at sea level.
If the researchers’ discovery is confirmed, the new form of ice will be called “Ice XVII,” a naming quirk that resulted from scientists terming the first two identified forms “Ice I.”