Removing the Drawbacks
New techniques and materials are being made every day to improve the way batteries are made. Not only are conventional electronic products the recipients of these developments, so are renewable energy products and electric vehicles. However, sometimes even these new developments have drawbacks.
Lithium-air batteries are one such example. Initially viewed as highly promising due to impressive output-to-weight ratios; they are now being viewed with caution. Such drawbacks include the amount of energy wasted as heat and expensive extra components needed to pump oxygen gas in and out of the battery.
Given the promise of the concept, it had to be completely rethought. An international team of researchers has developed a new battery concept based on lithium-air called a nanolithia cathode battery. The new tech can deliver the same performance as lithium-air without any of those pesky drawbacks.
The study, published in Nature Energy, describes a battery that is mainly a variation of the battery chemistry of lithium-air. The study aimed to tackle the primary problem of lithium-air, voltage mismatch of charging and discharging the battery. This constitutes a loss of 1.2 volts.
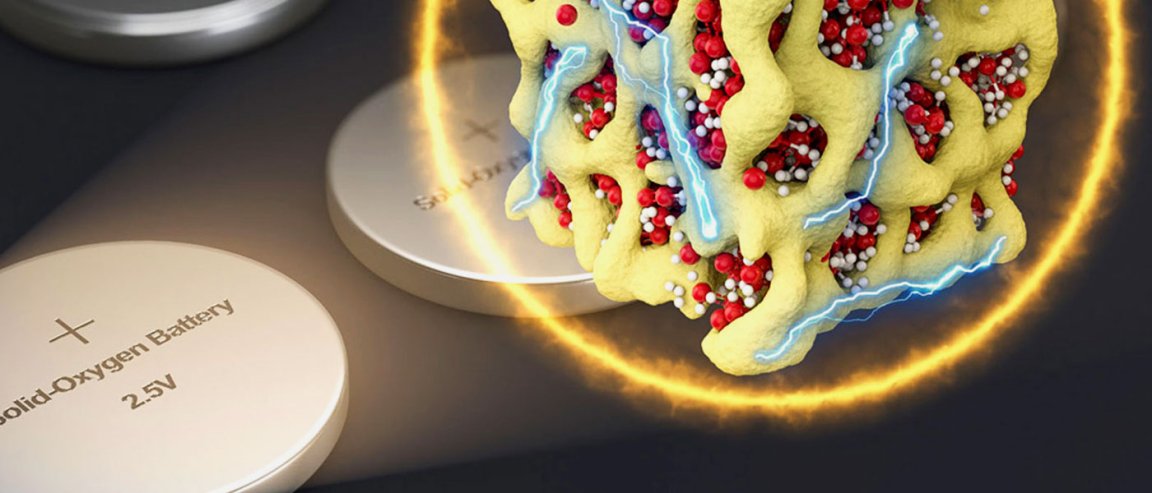
Sealed in a Glass
Conventional lithium-air batteries revert the oxygen they get from charging back into gaseous form and released back into the atmosphere after it is used. The nanolithia batteries dispense with this: the oxygen stays inside the solid, bound in the form of three different solid chemical compounds, Li2O, Li2O2, and LiO2, and mixed together in the form of a glass. The voltage loss decreases from 1.2 volts to 0.24 volts, so only 8 percent of the electrical energy is turned to heat. This also solves the issue of volume changes when the oxygen alternates between solid and gaseous forms, degrading the battery. This whole process is made possible by minuscule particles, called nanolithia which contain both the lithium and the oxygen in the form of a glass. This mix is stabilized by a matrix of cobalt oxide.
The end product is a battery that is cheap, scalable, and much safer than alternatives.