The New Periodic Table
And with a single announcement, millions of text books around the world have been made incomplete—out of date.
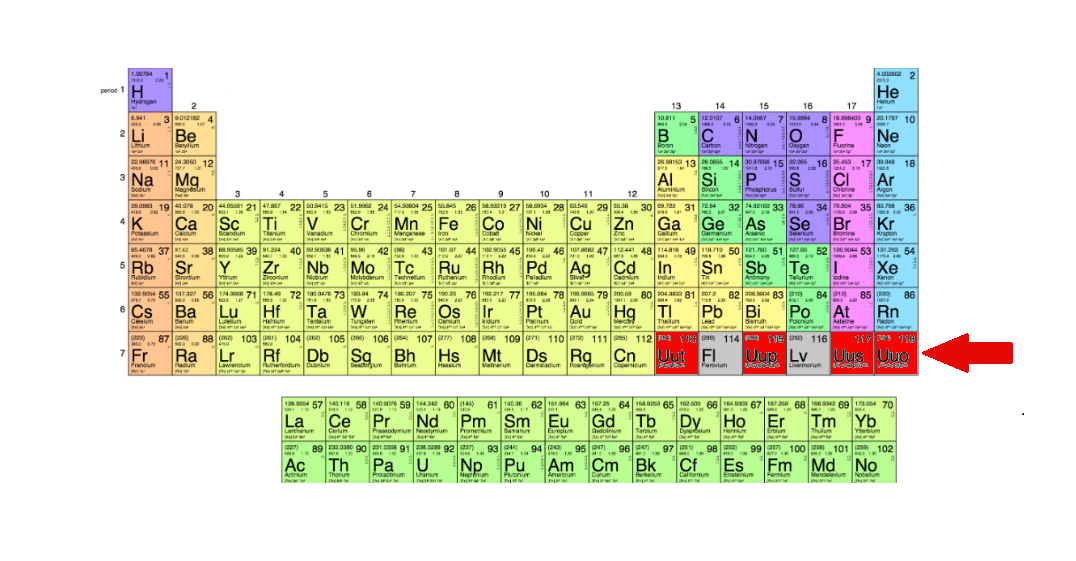
This comes as four new elements are added to the periodic table, finally completing the table’s seventh row. The elements, 113, 115, 117, and 118 were discovered by scientists working in Japan, Russia, and America. Now, the International Union of Pure and Applied Chemistry (IUPAC, the body that is charged with overseeing standards in the world of chemistry) has confirmed the finds.
Ultimately, these are the first elements to be added to the table since 2011. They were verified in an announcement released on Dec. 30.
At the time of the announcement, the IUPAC stated that a united Russian-American team of scientists, located at the Joint Institute for Nuclear Research in Dubna and Lawrence Livermore National Laboratory in California, had produced sufficient evidence to claim the discovery of elements 115, 117, and 118. However, the discovery of element 113, which had been claimed by the Russian and American team, went to a team of scientists from the Riken institute in Japan.
Ryoji Noyori, former Riken president and Nobel laureate in chemistry, notes that the achievement is one of the greatest accomplishments that a chemist can undertake. He stated in the release,“To scientists, this is of greater value than an Olympic gold medal”.
Unfortunately, we don't know the actual names of the new elements...because they haven't been decided yet.
The elements all currently have placeholder names; they will be officially named by the teams that discovered them sometime in the coming months. Professor Jan Reedijk, president of the Inorganic Chemistry Division of IUPAC, outlined the current status of the names, stating, “IUPAC has now initiated the process of formalising names and symbols for these elements temporarily named as ununtrium, (Uut or element 113), ununpentium (Uup, element 115), ununseptium (Uus, element 117), and ununoctium (Uuo, element 118).”
Ultimately, the new elements can be named after several different things: A mythological concept, a mineral, a place/country, a property, or a scientist.
Notably, all of the elements in question are synthetic. In other words, we don't find them naturally on Earth; rather, they were discovered by slamming lighter nuclei into each other and tracking the following decay of the radioactive superheavy elements.
A Table in Flux
To many people, this announcement may be a bit of a surprise. After all, the Periodic Table of Elements seems like it should be something that is unchanging. Many might assume that there shouldn't be new elements suddenly cropping up out of the blue.
However, this is based on a faulty understanding of how our universe (and science) works.
In truth, this isn’t the first table of elements. In fact, we once believed that all things in the universe were made from just Earth, Fire, Water, and Air (thankfully, we didn’t stick with that organizational structure).
Of course, today, the Table is a lot more solid and well established than it was when it was first developed. But that is because, at that time, many elements weren’t discovered yet. Conversely, these days, we already have an abundance of elements—meaning that most all naturally occurring elements on planet Earth have already been discovered and the Periodic Table has been mostly filled in. Mostly.
If a new element is discovered, it is generally the result of physicists smashing atoms together to see what happens i.e., a new element is only likely to be discovered in a particle accelerator (like what occurred here).
And notably, as this announcement reveals, new elements are being discovered, and it is leading us to a more complete picture of the things that comprise our universe.
Share This Article