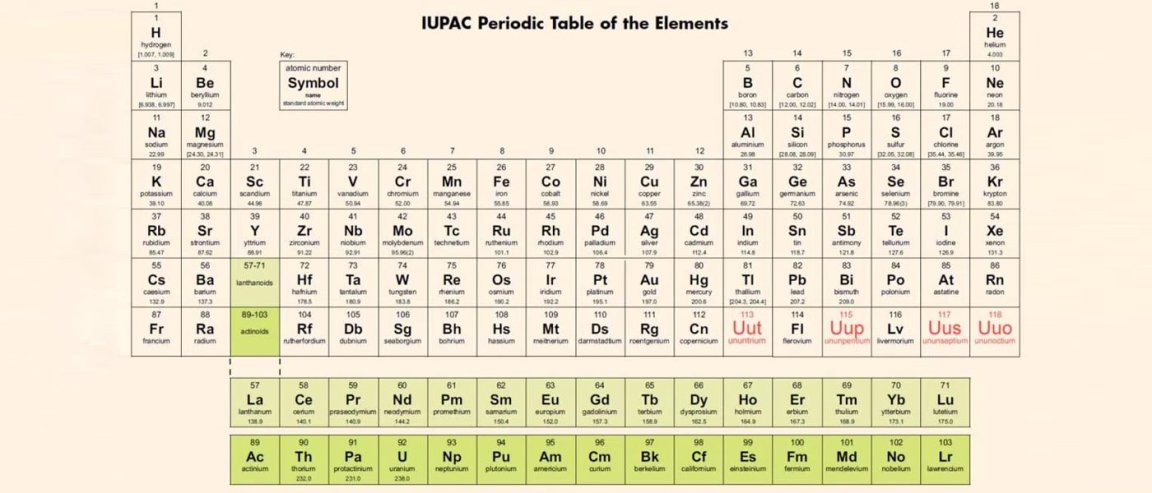
New Year, New Elements
The elements formerly known as 113, 115, 117, and 118 have been officially named Nihonium (Nh), Moscovium (Mc), Tennessine (Ts), and Oganesson (Og), respectively. With this confirmation, they can join their brethren on the periodic table.
“Following a five-month period of public review, the names earlier proposed by the discoverers have been approved by the IUPAC Bureau,” the International Union of Pure and Applied Chemistry (IUPAC) announced. IUPAC unveiled the discovery of these four elements as far back as January of this year, after which the research team behind each was tasked with naming its discovery.
Following tradition, the new additions to the seventh row of the periodic table received names that paid homage to either the region of their discovery or a person, and their suffixes reflected chemical consistency. Nihonium, Moscovium, and Tennessine are named after places where they were discovered: Nihon (the Japanese word for Japan), Moscow (Russia’s capital), and Tennessee (the U.S. state), respectively. Oganesson was named after Yuri Oganessian, a Russian nuclear scientist.

A Periodic Table in Flux
Perhaps the most interesting thing about these elements is how unstable they are. All four are radioactive with very short half-lives that last only a fraction of a second. If it weren’t for consistently progressing scientific and technological advancements, we wouldn’t be able to observe and study them as they aren’t known to exist outside of a lab setting.
Following IUPAC’s stringent approval process for naming the four new elements, the final step now involves transitioning to an updated periodic table, but it won’t be the first or last time we do that. As we discover more about our physical world and add to this ever-growing chart, we continue to gain a better understanding of how the universe works through the various elements that comprise it.
While most of our newly discovered elements don’t have much practical use, they can be viewed as stepping stones that will allow for the discovery of newer ones that could have practical applications in the future.