
There are various things we need for survival. Like water, food and energy. On the last point, our primary source of energy is the Sun, but our relationship with it can only be described as delicate. For instance, the Sun puts off energy in the form of heat. Too much of it — or conversely, not enough — would be detrimental to our existence. Thus, our planet has to be situated in just the right spot for life to be sustainable. Beyond that, how much do you really know about heat?
Heat — in our everyday language, in physics, specifically — really means internal energy, or the random motion of particles in matter. To put it more simply, the hotter the object, the more its particles move randomly. In physics, heat technically only refers to energy moving from a hotter object to a colder object. We know that heat always flows from an object that’s hotter to one that’s colder, but why can’t it happen in reverse? A hot cup of coffee gets cold because it transfers heat to its surroundings. Why can’t its surroundings transfer heat to the coffee to make it hot though?
The answer lies in the second law of thermodynamics, which states that the entropy — which, in a textbook, is typically described as disorder — of a system always increases. For example, your bedroom has the tendency to become more disordered with time (unless you clean it every single day, it will never remain clean). Now, when it comes to heat transfer, a more appropriate definition of entropy is the dispersion of energy. In simple terms, energy tends to scatter out to its environment. A hot cup of coffee, therefore, will scatter its internal energy to its environment until it gets cold (as a cool side-note, this phenomenon also says something important about quantum entanglement and the role it might play in the arrow of time).
Now, we can finally ask the question: “How is heat transferred?” Importantly, at times it is desirable to control the heat transfer between two objects. For example, we want ice cream to cool down and soup to warm up. We also want to maximize heat transfer between our stove and our food. A great example of controlling heat transfer is a thermos (or a beverage bottle). The thermos helps regulate the temperature of the liquid inside of it because it minimizes heat transfer between the liquid and its surroundings.
Now that that’s out of the way, to answer the question, we must then know how heat is transferred.
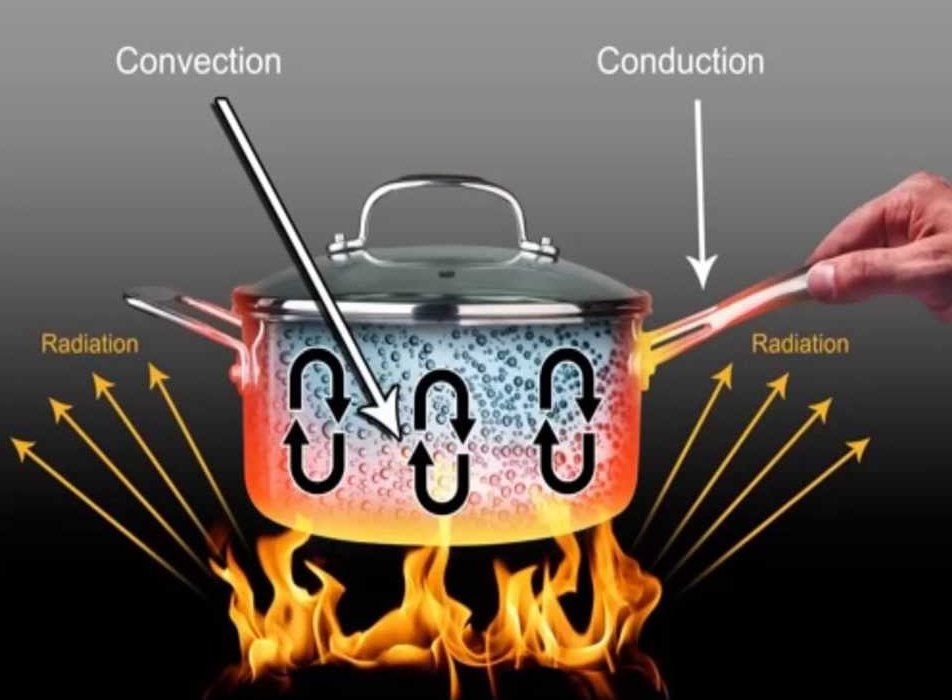
The Three Modes of Heat Transfer:

Conduction:
Conduction is simply the flow of heat following physical contact. If you touch a hot flat iron, you will get burned (heat flows from a flat iron to your finger because they are in contact with each other). Different objects conduct heat differently; certain things conduct heat very well (like metals), and some do not (like plastic). When we touch an object to feel its temperature, what we feel is not the temperature of the object, but the temperature of our skin. To expand, when we touch a cold object, heat from our skin transfers as a result of conduction to the object, making our skin colder. Thus, we “feel” that the object is cold. This method of sensing temperature is not accurate since, as I said, different objects conduct heat differently.
Convection
Convection is heat transfer due to motion of a fluid (liquid or gas). Hotter fluids become less dense and rise up, while colder fluids become more dense and go down. When we heat water in a pot, the water at the base of the pot will become hotter first. When this happens, the hotter water at the bottom will go up, replaced by the colder water above. This, in turn, will get hotter and then go up, replaced again by the water on top. This way, heat is transferred to all of the liquid in the pot evenly.
Radiation
Radiation is heat transferred by electromagnetic waves. These electromagnetic waves have energy, and when absorbed by the receiving object, they heat the object up (this is how the Sun heats up our planet, and how microwave ovens heat up our food). However, radiation is different from the other two methods because it doesn’t require a medium for heat transfer between two separate objects.
Back to The Thermos Bottle:
The technical name of a thermos is a vacuum flask (you will know the reason why, shortly). To keep your drinks hot or cold, it minimizes heat transfer due to conduction and radiation. Convection is not included because convection keeps the temperature in a liquid even. You don’t want to drink something that is hot on the first gulp and gets colder and colder until the last one.

A vacuum flask is nothing but a bottle inside a bottle separated by a vacuum. This is because with a vacuum surrounding the bottle, conduction is effectively minimized. There is nothing in contact with the bottle except the part that holds the inside bottle in place. But there is still a small bit of contact, which makes the inside bottle conduct heat to a small degree. If, however, you want to accelerate the process of cooling your drink down using a freezer (by speeding up the transfer of heat, instead of minimizing it), you have to surround your drink with something that conducts heat very well, like a wet paper towel, because water conducts heat much better than the air inside of a refrigerator.
By doing this, heat transfer is not entirely eliminated because of radiation. Every object radiates heat to some degree (depending on how hot the object is). A hot liquid in the inside bottle will still radiate heat, or the outside bottle will still radiate heat toward the liquid inside the bottle. To minimize this, the surface is coated with silver. Silver prevents electromagnetic waves from passing, thus it keeps the radiation at bay (this is the principle used in Faraday cages).
With these techniques, our drinks are kept hot or cold, thanks to a little understanding of heat transfer and the laws of thermodynamics!
This article is listed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. However, FQTQ does not own the rights to the images that are attached.. Learn more about our republishing policy here.