Oral Therapy for Neurological Disease
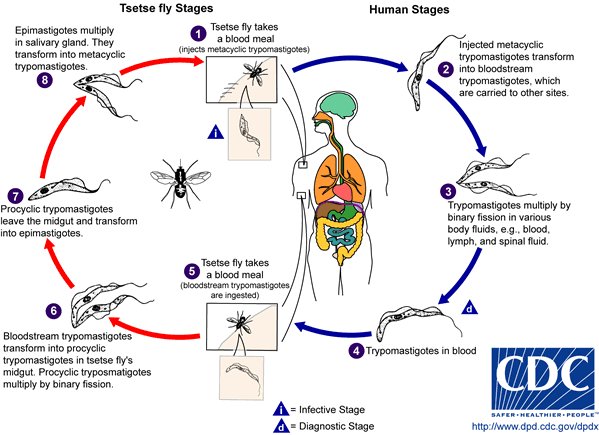
Sleeping sickness sounds like something from a fairy tale, except it isn’t, especially when you know how much pain and suffering this disease causes. Also known as human African trypanosomiasis, sleeping sickness is a parasitic disease that comes from the bite of a tsetse fly, which infects the central nervous system. It’s symptoms include fever, headache, joint pains, and itching. It gets worse after weeks or months, when a patient starts manifesting neurological symptoms, such as sleeping problems, confusion, poor coordination, and numbness.
Currently, treatment is effective only when sleeping sickness is detected early — i.e., before the onset of the neurological symptoms. The usual method involves a combination of pills and intravenous infusions. That might soon change, however, as researchers from the Drugs for Neglected Diseases initiative (DNDi), a non-profit based in Geneva, Switzerland, developed a method that relies only on pills. Clinical trial results presented on October 17 at the European Congress on Tropical Medicine and International Health in Antwerp, Belgium, suggest this oral method to be effective, and could potentially eliminate the deadly neurological disease within a decade.
The pill called fexinidazole was able to cure 91 percent of patients suffering from a severe sleeping sickness. Although the usual combo therapy of infusions and pills cured 98 percent, fexinidazole was able to treat 99 percent of the patients who were still in an early stage of the disease. Usually, these would have to undergo a spinal tap before to determine the viability of infusions, but the revolutionary method makes it simpler. If approved, the researchers are convinced that the relative ease with which fexinidazole is given could save more lives than current methods.
Simple and Cost-Effective
Prior to the combined therapy, the only treatment for sleeping sickness was a toxic arsenic-based drug that ended up nearly as fatal as the disease itself, killing one in 20 patients. Sleeping sickness incidence, according to the World Health Organization, has dropped to around 2,200. However, the combined treatment method still doesn’t come easy. It’s tedious, costly, and requires materials difficult to come by, especially in the African areas where sleeping sickness remains prevalent.[infographic postid=”18884″][/infographic]
“It’s not just the person with sleeping sickness, it’s the family that takes care of them during years of this neurological, very serious disease,” Philippe Büscher, a sleeping-sickness expert at the Institute of Tropical Medicine in Antwerp, Belgium and was not part of the study, told Nature. “Whatever money they have, they’ll spend on this instead of anything else.”
The DNDi continued to search for a better alternative, and in 2007 they stumbled upon fexinidazole, a drug that was previously shelved by Paris-based pharmaceutical company Sanofi. The clinical trials were conducted in the Democratic Republic of the Congo and the Central African Republic, areas with high number of sleeping sickness cases. The researchers estimate that, once approved by the European Medicines Agency, developing the new therapy would only cost around $50 million. That might seem a lot, but it’s only a fraction of what pharmaceutical companies usually spend on new drugs.
“This is a success,” Büscher said, “but it is not the end.” Indeed, the DNDi researchers are currently working on an even better option, one that could treat sleeping sickness with just one dose.