

Born in 1885 to Christian Bohr – a professor of physiology – and Ellen Adler Bohr in Copenhagen, Denmark, Niels Bohr made numerous contributions to the field of physics over the course of his life, up until his death in 1962.
After studying philosophy and mathematics at the University of Copenhagen in Denmark, he conducted a series of experiments dealing with the properties of surface tension. These experiments were performed using his father’s lab, which was located at the university.
Following his initial experiments, the essay that he produced on his findings won a competition that was sponsored by the Royal Danish Academy of Science and Letters (this honor was quite an accomplishment at the time). This prompted his transition from philosophy to the field of physics – a subject he would soon find himself taking quite the liking to.
In a way, Niels Bohr was the pioneer of quantum physics (second only to Max Planck himself). At the very least, he was one of the many fathers of the field. He received the Nobel Prize in physics in 1922 for his work in investigating the structure of an atom, which effectively began a half decade long inquiry into the field of quantum mechanics – a field that is nearly as mystifying now as it was then.
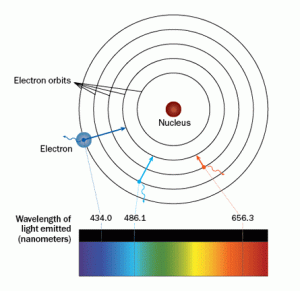
After this time, Bohr began work at Manchester University, where he adapted Rutherford’s nuclear structure to adhere to Max Planck’s quantum theory. Based on his model of the atomic structure, which was published in 1913, he is credited with being the first person to suggest that electrons travel in an orbit around the nucleus of an atom.
Soon after, he also introduced fellow scientists to the idea of electrons being able to drop to a higher energy level to a lower one. In the process of this transformation, a photon of discrete energy is emitted.
Furthermore, he was able to determine the chemical properties of each element largely by determining how many electrons exist in the outer orbit of its atoms. All of his work became the basis of quantum physics, which is the field of study that deals with the interactions between the laws of physics and subatomic particles that make up matter on a microscopic scale.

Unbelievably, Bohr had direct connections to two of the most famous physicists in history. Bohr and Albert Einstein spent many hours debating the core principles of quantum physics. Einstein himself, who came up with two of the most celebrated theories in physics, general and special relativity — was not much of a fan of the budding field. In fact, he thought it was counter intuitive to the subtle way in which the universe is put together.
He saw quantum mechanics as little more than rolling a set of dice – and to him, “God” does not play dice (which was not a statement of faith, but an assertion about how Einstein saw the physics of the universe). Bohr also worked with Werner Heisenberg, who was his assistant in Copenhagen from 1926 to 1927.
It was in this small space of time that Heisenberg began to put the pieces together for his uncertainty principle, which states that the more precisely the position of a particle is determined, the less precisely the momentum can be known (and vice versa).
Besides his work in the field of physics, he also had close ties to the government. He was a participant in the infamous Manhattan project (his code name was Nicholas Baker), where he helped construct the first atomic bomb. He first explained the process under which, uranium fission could create a powerful blast by splitting the atom with a few pounds of uranium.
Though nuclear weapons are now known to be quite destructive, he strongly believed that atomic secrets should be shared among the international scientific community. He even visited president Roosevelt in hopes of convincing him to share pertinent details of the Manhattan Project with the Soviets during World War II. After the war was finished, he continued to be an advocate for the peaceful use of nuclear energy. This fight continued until his death of heart failure in 1962.
Fun fact: The element Bohrium (whose properties were first predicted by Bohr himself) is named after him.