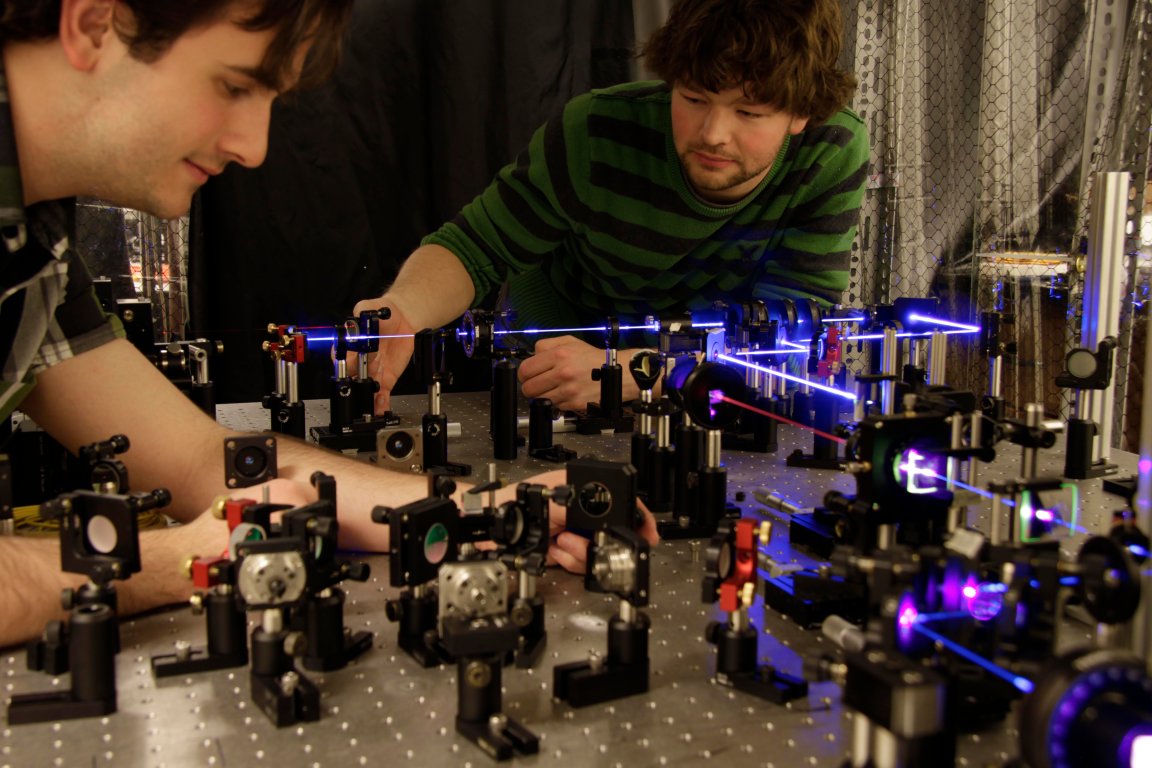
The Heisenberg Uncertainty Principle has been a staple (and an annoyance) in quantum mechanics ever since Heisenberg wrote down that irritating formula in the early 20th century. In short, it states that you cannot know both the location and the momentum of particles. The more certain you are of one, the less certain you are of the other.
Now, with the world making leaps in quantum technology (and the possibility of a new technological revolution looming), knowing exactly how precise a measurement you can obtain is very important. It turns out Heisenberg might have been wrong.
Now, before you mourn the loss of every uncertainty joke we’ve created in the last century, it is important to know that it still applies, just not as strongly as Heisenberg originally stated.
Instead of taking one large measurement of a particle (which disrupts the system and yields a ton of uncertainty), a team of scientists from the University of Toronto took a bunch of small measurements in an attempt to interact with the system as little as possible. Their results were unprecedented, when these measurements were stacked together, these scientists were able to get a more accurate measurement of their test subject than the Heisenberg uncertainty principle allows. Their study shows a new mathematical measurement-disturbance ratio created by Dr. Masanao Ozawa in 2003 is more accurate.
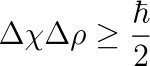
Their findings were published in the journal “Physical Review Letters” and also presented at the Optical Society’s Annual Meeting, Frontiers in Optics in September 2013.
Only time will tell if Heisenberg’s original formula stands the test of scrutiny as scientists from around the world attempt to replicate these results.