Nature-inspired
Nanotubes are an important part of the next generation of technology. Hollow tubes that have diameters of only a few billionths of a meter, nanotubes have been used to make and improve everything from artificial retinas to transistors.
But since they are small, you have to make a ton of them. We’re talking millions. And making millions of nanotubes with the exact same diameter presents a very real challenge. Until now.
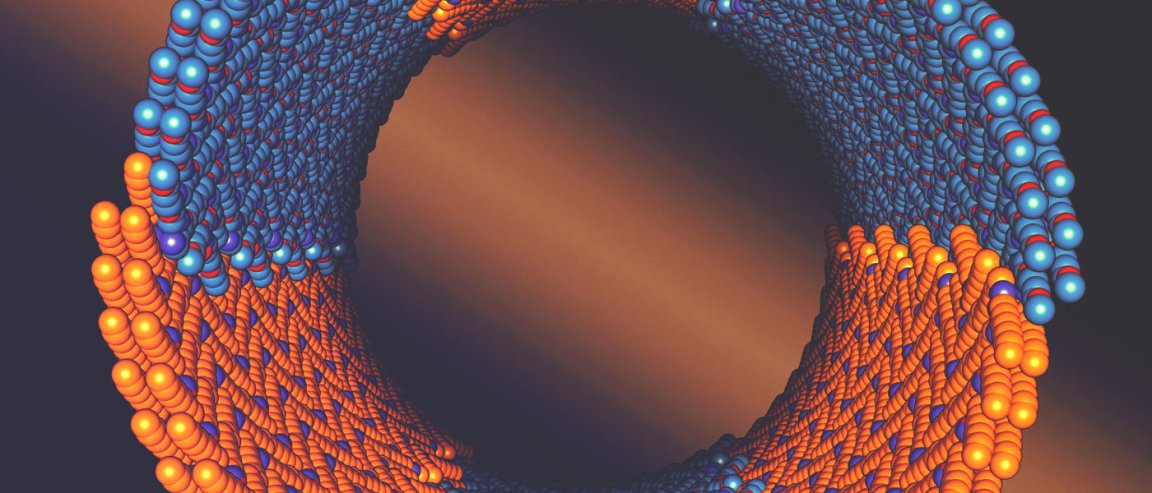
Researchers at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) have recently announced that they have discovered a family of nature-inspired polymers that, when placed in water, spontaneously assemble into hollow crystalline nanotubes. What’s more, the diameters can be tuned, producing nanotubes five to ten nanometers in diameter. This depends on the length of the polymer chain used.
Notably, these new structures could be used in order to deliver cancer-fighting drugs inside cells or to desalinate seawater.
New Design Principle
The breakthrough by the scientists reflects not only the discovery of the polymers, but also the new design principles on how these things were formed.
The study focused on a particular type of polymer, peptoids. Specifically, the diblock copolypeptoid. This peptoid is made up of two peptoid blocks, one hydrophobic and one that’s hydrophilic. When they meet in water, they crystallize and form rings.
Close inspection of the tubes, with the help of the Cryo-electron microscopy imaging, showed that the structure of the nanotube is composed of these rings stacked together, forming a striped pattern.
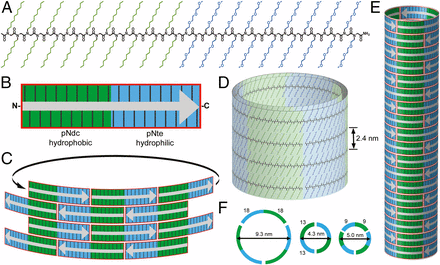
The peptoids arrange themselves in a brick-like pattern, with hydrophobic blocks lining up with other hydrophobic blocks, and the same for hydrophilic blocks. The two peptoid blocks are chemically distinct, yet almost exactly the same size, which allows the chains to pack together in a very regular way. This allows the nanotubes to assemble themselves without the usual nano-construction aids, such as electrostatic interactions or hydrogen bond networks.
“…[I]f you can control the diameter of nanotubes, and the chemical groups exposed in their interior, then you can control what goes through—which could lead to new filtration and desalination technologies, to name a few examples,”says Ron Zuckermann, who directs the Biological Nanostructures Facility, where much of the research was conducted.