
Carbon Product
How did volatile elements, like carbon, manage to stay in the Earth’s mantle?
According to planet formation theories, any volatile element should have boiled away during the Earth’s molten days some billions of years ago, or should have melded with the earth’s core. Instead, carbon and the other elements that made life possible, stayed in the planet’s mantle.
How did carbon life on Earth begin? Scientists from Rice University stumbled upon a (literally) earth-shattering, but plausible answer.
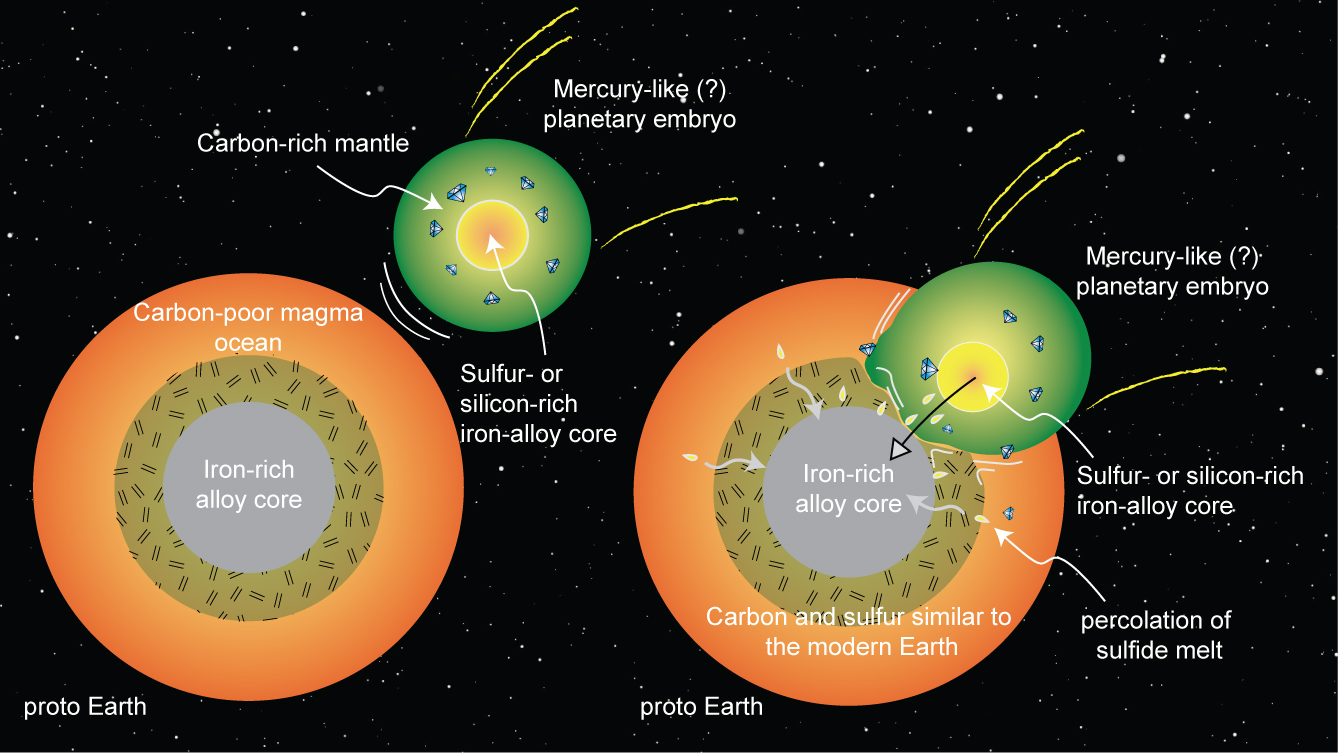
Petrologist Rajdeep Dasgupta proposes that the Earth could have been hit by an embryonic planet, similar to Mercury, some 4.4 billion years ago.
Ratio of Elements
The researchers at Rice were not convinced by the popular theory that meteorites added the volatile elements when the Earth’s surface was a lot more stable.
They tried a different scenario.
Moving away from metal with strong affinity for carbon, the usual supposed initial core composition of planets, the researchers opted for sulfur-rich and silicon-rich alloys, mimicking the cores of Mars and Mercury. “It was a compositional spectrum that seemed relevant, if not for our own planet, then definitely in the scheme of all the terrestrial planetary bodies that we have in our solar system,” says Dasgupta.
The researchers recreated the probable conditions of the collision in a high-pressure, high-temperature experiment. The result: carbon would have stayed in the mantle if the core was rich in silicon or sulfur. And this would have been possible if an embryonic planet with a silicon-rich core collided and combined with the Earth’s own core.
If this is true, the answer to the question of what happens when worlds collide may turn out to be: life.
The study was published in the journal Nature Geoscience.