GAME CHANGER
A newly discovered antibody can dramatically cut down on the visible signs of Alzheimer’s disease seen in the brain, marking a possible game changer in the efforts to prevent the disease.
To back up a bit, a common cause of dementia, Alzheimer’s disease is considered to be one of the most widespread medical crisis facing our generation. Yet it is also one of the least understood. In the United States, Alzheimer’s is estimated to be ranked as the third leading cause of death—right behind heart disease and cancer—for older people.
Researchers are continuously looking for new treatments for the disease. Recently, scientists discovered that mefenamic acid was able to reverse the symptoms of Alzheimer’s disease in mice. Now, another group of researchers are testing a new drug—this time on humans—that will reduce the proteins clumped in the brains of Alzheimer’s patients.
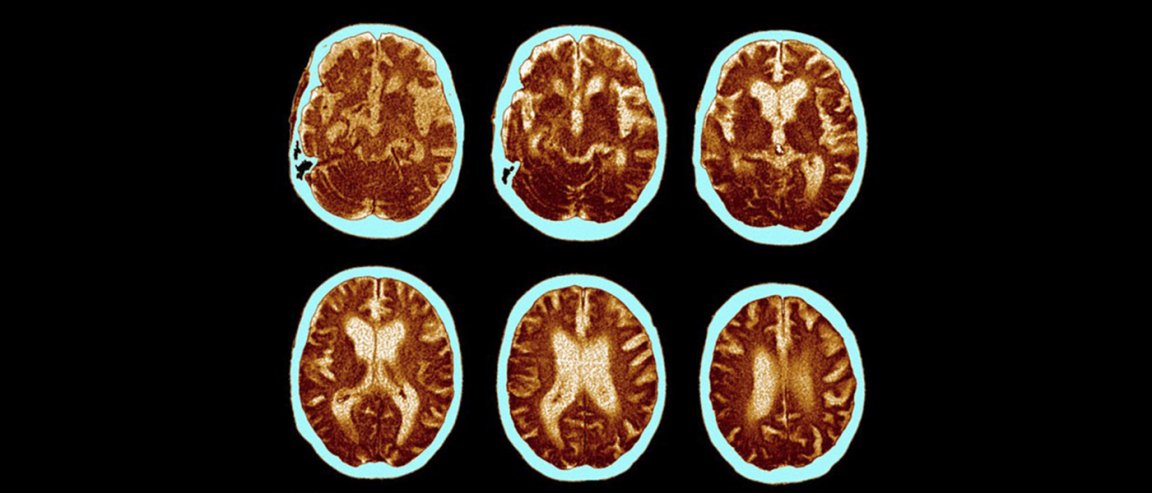
PLAQUES IN BRAIN
The drug, described in the journal Nature, is called aducanumab, and it targets the toxic amyloid beta plaque, which are tangled clumps of protein that formed in the brains of patients with Alzheimer’s. According to the researchers, the “human monoclonal antibody” prompts the immune system to selectively target and clear the aggregated plaques, which, in turn, could slow down the disease.
Pharmaceutical company Biogen, which funded the trial, said the antibody has been tested in both animal models and a small group of 165 patients with prodomal and mild Alzheimer’s disease. After a year of study, the researchers found that virtually all the amyloid plaques appeared to have gone in patients that were given the highest doses of the drug.
However, the study found that the patients on the highest dose of the drug—10 milligrams for every kilogram of body weight—were also more likely to experience side effects, which can lead to the swelling of the brain.
Dr. Roger Nitsche, president and founder of Neurimmune, a Swiss biotech group that discovered the medicine, described the effect of the drug as “unprecedented”—albeit one that still needs further trials.