A Stanford University research lab has been developing not one but two solutions to pressing technological problems: making clean hydrogen fuel and rechargeable zinc batteries for renewable energy farms
New Materials
Long touted as a clean alternative to gasoline, hydrogen-powered cars have been offered since last year, but let’s face the problem: cheap and clean hydrogen fuel is yet to be developed.
Making hydrogen fuel is not emission-free, the process does release CO2 into the atmosphere. A possible solution is making the photovoltaic water splitting– electrodes that split sunlight into constituent parts. But conventional solar electrodes made of silicon quickly corrode when exposed to oxygen.
The study, published in Science Advances, proposes the use of bismuth vanadate in solar cells. The material absorbs sunlight, generates modest amounts of electricity, and is resistant to corrosion.
To offset the shortcomings of using bismuth vanadate, which include letting much light pass through the material, the researchers created microscopic arrays containing thousands of silicon nanocones to increase the light-trapping ability of the solar cell.
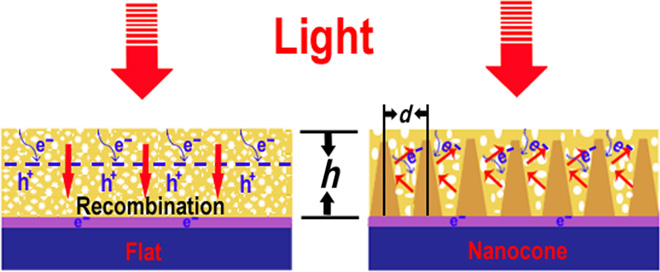
In testing the new solar cell, the device had a conversion efficiency of 6.2 percent, matching the theoretical maximum rate for a bismuth vanadate cell, thereby suggesting how much our electricity conduction can improve.
Changing Faces
In a second study published in Nature Communications, researchers proposed a new battery design that could allow both wind and solar farms to store excess electricity produced.
Zinc-nickel batteries have been studied for possible use in grid-scale surplus energy storage. But dendrites that form on zinc electrodes during charging cause short circuits.
The researchers propose a change in battery design: making barriers. Conventional batteries have the zinc and nickel electrodes face one another, which is why zinc dendrites reach the nickel electrodes.
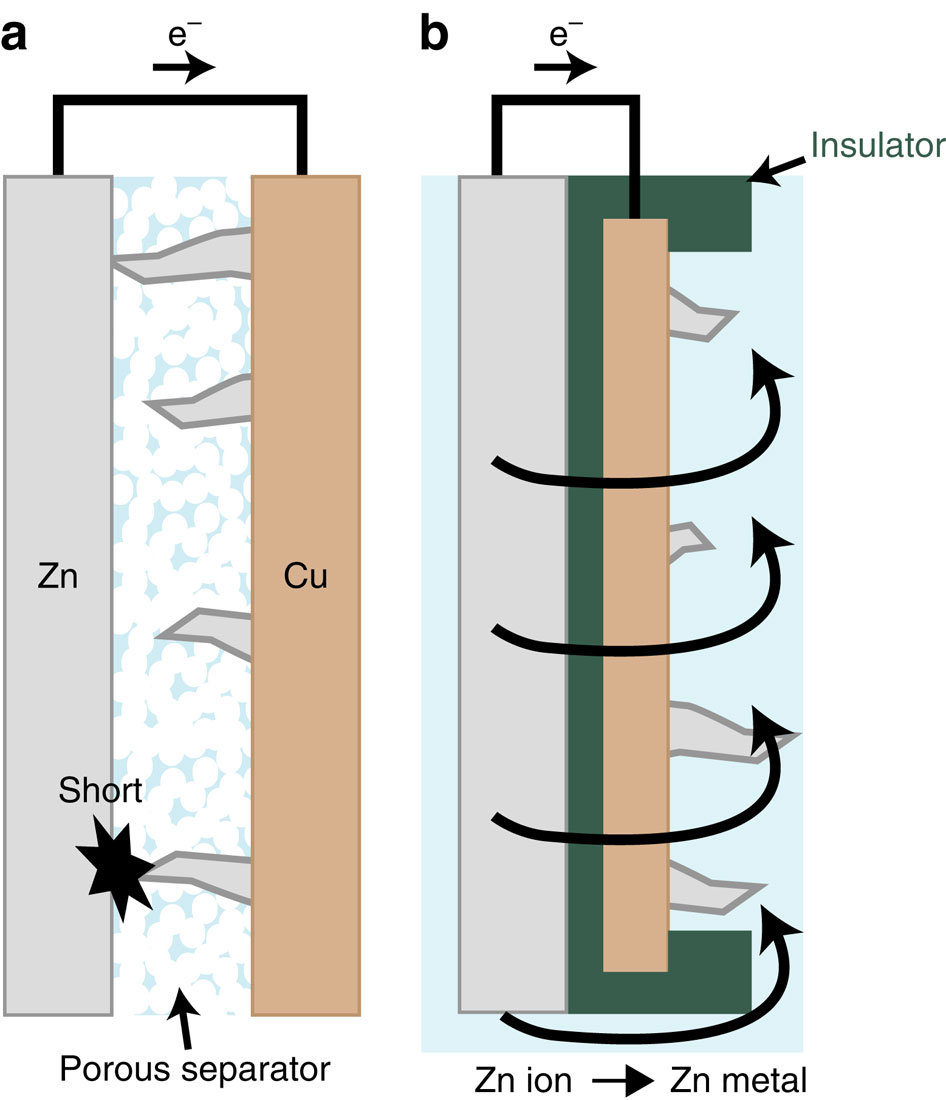
By separating them with a plastic insulator and wrapping a carbon insulator around the edges of the zinc electrode, the dendrites form in the other direction and never reach the other electrode. To demonstrate stability, the researchers successfully charged and discharged the battery more than 800 times without shorting.
Ultimately, it is innovation like this what will usher a new age of progress—ubiquitously.