
Tough bonds
Scientists from the American Chemical Society (ACS) have just released a study published in ACS Central Science that details the creation of a completely new chemical bond between two elements. Those two elements are iron and bismuth, both previously thought impossible to bind chemically. It turns out, all they needed was a little bit of pressure — just equivalent to that within Mars’ core
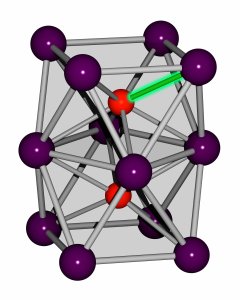
The ACS researchers quite literally forced their way through the elemental antipathy between iron and bismuth. The result is FeBi2, a new material with an iron-bismuth bond previously impossible without advances in high-pressure techniques. It also didn’t exist in any known substances.
Attempts to mix the two elements only yielded significant results at pressure levels around 30 GPa, and it was synthesized at 1,500ºK (2,240ºF). The researchers found that FeBi2 remains stable even when pressure was lowered to 3GPa, but not when lowered to Earth’s atmospheric pressure levels (about 30,000 times lower).
Exploring its potential
Since FeBi2 is a new material, its actual potential hasn’t exactly been discovered yet. Its makers surmise that it can help in the development of brand new magnets and better superconducting materials.
“Ongoing work is focused on the scale-up of this reaction with a unique, highly specialized press capable of reaching the required pressures in order to enable studies of the electronic properties of this fascinating new material,” the study says. This is intriguing, to say the least.
In a world where energy is becoming easier to harness but not utilized as efficiently as possible, research in improving energy production is prolific. Among these, the development of better superconductors is highly popular. Superconductors improve energy usage by limiting (and perhaps, in the future, negating) loss due to transference.
For now, what’s certain is that this technique that produced FeBi2 opens up a world of possibilities for material sciences.