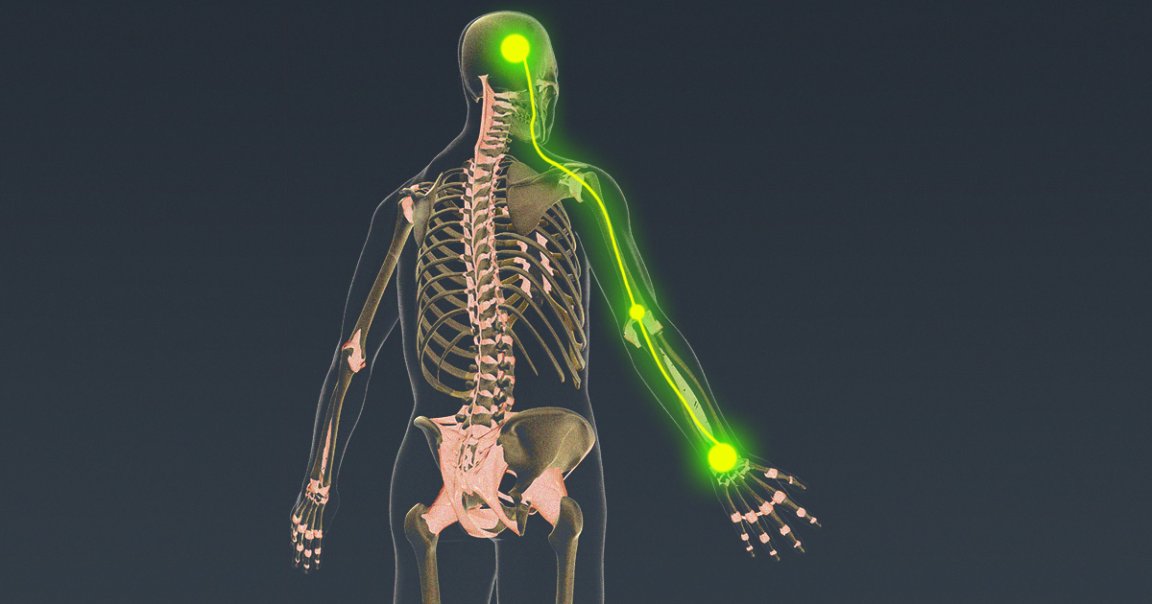
A team of scientists recently published intriguing research on a tiny, splinter-like brain implant that doctors can slide deep into the folds of the brain and use to restore both muscular control over and sensation from a paralysis patient’s limbs.
In a pair of scientific papers about research on three patients, one published in the journal Brain Stimulation and the other in Frontiers in Neuroscience, scientists led by bioelectronic medical engineer Chad Bouton of the Feinstein Institutes for Medical Research and Northwell Health found that the implant can record and decode the brain’s activity, route it through a computer instead of the damaged spinal cord, and use it to stimulate muscles directly — essentially bypassing a patient’s damaged nervous system and letting them control their hand again.
But that’s not all. The system was also able to collect data about finger position and pressure in order to stimulate the sensory region of the brain, providing the subjects with a sense of touch and proprioception about their own limbs.
In short, the implant can help people move and feel their limbs by circumventing whatever injury or condition had been blocking their brains from communicating with their bodies. Now, Bouton says, his team has clearance from the FDA to conduct an ambitious clinical trial that tests both capabilities — and grant new levels of independence to people with paralysis.
Futurism caught up with Bouton after his two recent papers went live to talk about his goals and vision for the neural implant. Here’s our conversation, edited for length and clarity.
This implant aims to restore both sensation and muscular control. Where did that idea come from?
Chad Bouton: There of course has been great work in stem cells and techniques to try to promote neural regrowth and reconnection. And obviously, that work progresses, but there are still challenges. I had the really nice opportunity in my career, early on, to be involved with the BrainGate program. The first time we put these electrode arrays in the brain, in the motor cortex specifically, patients could move cursors. All of that really made headlines and we broke new ground and milestones were met, but none of the patients were able to move. It just wasn’t the focus of the study. So a few years later, I had the idea that if we can decipher or decode signals in the brain from the motor area, if we can understand that someone’s thinking about opening their hand and moving different fingers and closing their hand, well, then why can’t we now reroute those signals around the injury or the damaged part of the nervous system, and then allow real-time stimulation of the muscles?
We set out to do that. We had the first clinical study in which a young man who had been paralyzed for about four years from a diving accident became the first paralyzed human to use a brain implant to regain actual movement of his own hand, and not a robotic arm, but his actual hand. That was a medical first and I dubbed the phrase “electronic neural bypass.” If we can do that in one way, why couldn’t we do it in the other direction or make it bidirectional? So we’re calling what we’re working on bidirectional neural bypass. We can now even take information — pressure from the fingertips — with these little thin sensors and then perhaps actually reroute those signals around the injury and send the signal back into the brain. And that’s the big part of what we just did.
One is about recording and decoding, using this very thin, minimally-invasive electrode that you can put in the brain and record the code. And we can also stimulate and create sensations on the fingertips. The fingertips are so important, right? Think about all the things we do every day, clothing ourselves, and doing fine motor skills. We started in one place and then realized, “Wow we should try to keep going and tackle the big problem.” There are over 100 million people living with some form of paralysis around the world, and its leading causes are stroke and spinal cord injury, or traumatic injury, and it’s a big problem that’s still unsolved.
At a general level, what input is this implant taking in and how is it actually translating that into restored perception and muscular control? Does it matter where the injury is?
Bouton: Let’s do an example. So if someone had a car accident and they had a spinal cord injury, the injury is between the brain and the muscles. We know the spinal cord is kind of the information highway. It’s nothing like the brain, but it’s a whole network in itself. Quite complicated. So your question about where we put the implant — these two big papers just came out where we showed that we can use a very thin electrode, a depth electrode, or sometimes called a stereo EEG electrode, that’s less than a millimeter in diameter. The surgeons drill really tiny holes in the skull and slide it in. We’re trying to target a little bit deeper than all of our previous work. And we’re trying to go a little deeper and down into the folds of the brain, the sulci. It’s been known for a while that the [parts of the brain corresponding to the] fingertips are actually a little more down into the folds.
We focused on the fingertips and creating or restoring sensation. That’s the ultimate goal. We showed for the first time that you can stimulate down into down in these deeper areas in the folds of the brain and give highly focal sensations, or percepts, in the fingertips. So people reported, “Oh, wow, I feel a little tingling or a little buzzing or a little pressure on the tip of my thumb or the tip of my index finger.” And we did that consistently. That was huge. And then at the same time, in three participants, we recorded signals as people moved individual fingers. We found that we could not only tell which finger they’re thinking about or moving individually, like say thumb or middle finger, but we can also pick up interesting signals from what’s called the white matter of the brain. We could get it up to 91 percent, even better sometimes, decoding accuracy.
Why is that important? Well, think about the paralysis example. Somebody wants to move their hand again. So we use the thin electrodes, we record from that area to tell, “Okay, they want to open or maybe do a pinch grasp; pick up a small object or a grape or something they’re eating.” And we decode and say, “Okay, we know what they want to do.” We send it to a computer and the computer sends a signal to muscle stimulators. And we’ve developed these really cool gold electrode arrays that you put right on the skin. We stimulate the muscles directly based on their thought patterns. And that’s how you reroute the one direction.
We now have FDA clearance to do a new study where we take sensory information, where someone picks up a grape, or whatever it might be. And we have these little thin film sensors that we’ve developed. We put them on the fingers. They’ve been stretched like the skin or they’ve been flattened just like your skin does when you touch an object, and they generate an electrical signal. We send that back to the computer and the computer sends stimulation patterns back into, again, the folds of the brain. Well, that’s the next thing we want to do. We have FDA approval to do that study next and close the loop, if you will.
Is there any sort of signal delay, since you need to go from neural signal to implant to computer to muscle stimulation? What about the other direction?
Bouton: The team has actually worked very hard to reduce that delay. There’s always a delay, even in someone who does not have a spinal cord injury. Signals take a little time to travel. We’ve developed AI algorithms that have a very short, very fast response. We know within less than a second what the person wants to do. And that’s very usable. One of the challenges with EEG on the scalp itself, is that there are still some delays. And with this, by putting the implant in the brain and by recording from the brain directly, by using our new approaches, we can really keep those delays pretty short. Which is important, right? I mean, think about it. If you want this to be functional and actually usable, you’ve got to reduce those delays.
So I know you’re in early days: you’re planning the next study, and this research is based on just three patients. But is there a commercial goal here or a broader goal to get this tech into the clinic?
Bouton: I think that we still have to study this approach. It does have the big advantage of being minimally invasive. But we still have to develop what we call a chronic or a long-term version that can be left in for longer periods. And then we also have to continue to map a little bit more on where exactly we want to record and stimulate from. These two papers should shed some significant light on that, but we still want to do more.
I would not rule out that we could start contemplating commercializing some version of this kind of technology, but we’re probably still several years out.
The reason I ask about future direction is because the FDA did grant human experimentation approval to another company called Synchron. They’re also focused on minimally invasive tech and built an implant that actually stays in the brain’s vasculature. Is this focus on minimally-invasive procedures a growing trend in the field?
Bouton: Absolutely. Yeah. I know their work quite well. We’re aware of each other, obviously, and what they’re doing is great. I think that they have a similar goal, making it minimally invasive and making BCI more available to more people, which I applaud. That’s absolutely what we want to do. I think that it’s also about where in the brain can you place these electrodes. We’re trying to make sure we can reach as many important areas as possible, but safely. We want to reach areas like the hands and the fingers and the fingertips, and we want to reach the feet and legs. That’s why we’re mapping and getting a little deeper into the brain and trying different areas and going into the folds. That’s super important because you can now help an even broader range of people. If you can get into some of the speech processing and speech generation areas of the brain or even hearing or vision. The point is we need to look at these areas and reach as many areas in the brain as we can to help because there’s a wide range of conditions, as you know, that we can treat with this kind of technology in the future.
There’s a pretty wide diversity in the sort of patients who theoretically benefit from this, ranging from diabetes patients to people with spinal cord or brain injuries. How universal would you say this specific kind of implant and treatment could become across different kinds of injuries or conditions? Would expanding it take fundamentally different tech, or is it just a matter of further mapping the brain?
Bouton: It depends on what part of the brain you’re trying to reach. The question as we map more is going to be “Do we have the resolution?” Are there enough electrodes? Are they close enough in distance? Do they have enough precision? We do think this approach has a lot of appeal because you can reach deeper areas more safely. And also we can use the same idea of recording and stimulating because we know that you can do that in different parts of the brain. That’s already known.
There’s a lot of science that has to be figured out, but there has already been work done in terms of stimulating the deep brainstem. They also use this kind of depth electrode, where you stimulate the brain for Parkinson’s. Now, there have been studies on stimulating deeper structures for even obsessive-compulsive disorder and depression. But recording — a lot of that has been focused on motor area, like what we’ve done. In humans, there haven’t been as many recordings from as many different sites as we’ve seen with stimulation. So there’s a lot of room for more studies. I think we’re going to learn a lot. And I think it’s going to open up even more doors, quite frankly.
Anything else you want to talk about?
Bouton: We always try to think about the pathway to the patient. How do we take scientific and technological breakthroughs in the lab and how could we take them along the whole pathway, all the way to the patient? That’s part of the mission, part of being in a healthcare system. We’re very focused on the patient. But even in my lab, with all of our collaborators, I think we all have the same mission, which is to try and get this out to the clinic and get it to the patient.
So we think about the pathway to the patient, and what all the roadblocks are. You know, what are the boulders in the pathway and how do we anticipate those? How do we keep navigating this, make it safe. It is all about quality of life. That’s the other general comment I would make. We’re trying to just do everything we can to improve quality of life, wherever and whenever possible, and increase independence. I’ve had a lot of participants say to me, “I just want to be able to do more things on my own without having to ask for help.”
That’s really resonated with me over all the years. And that’s what we’re trying to do: help people be able to do more things on their own, without having to ask for help. It kind of puts it in a different perspective.