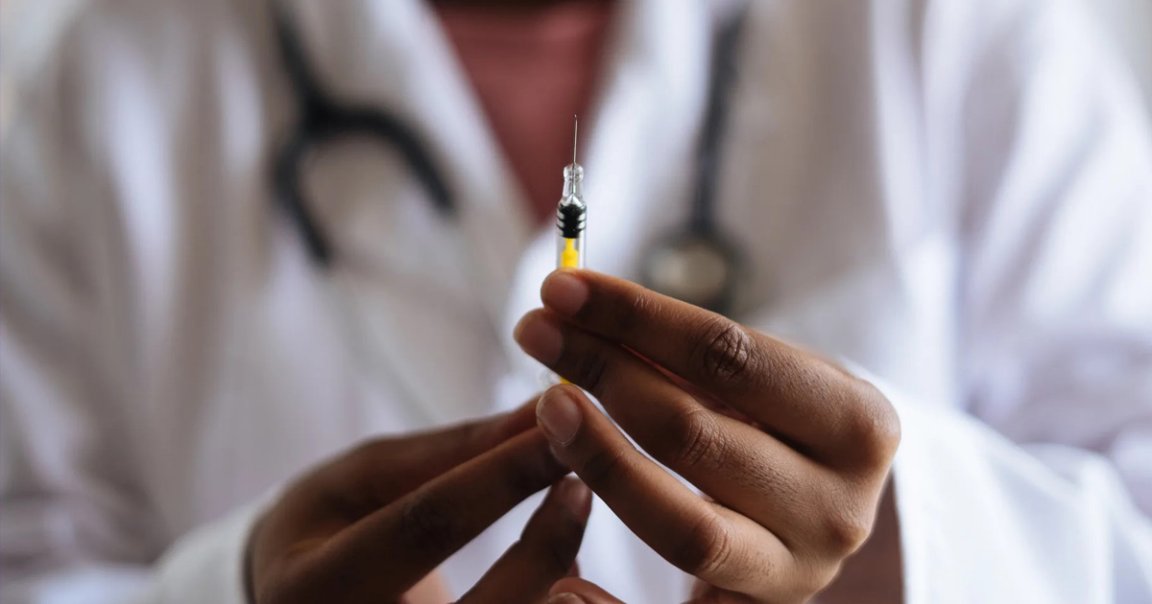
The UK has officially licensed the COVID-19 vaccine developed by multinational pharmaceutical company Pfizer and German partner BioNTech.
The country will begin vaccinating high risk groups as soon as next week with 800,000 initial doses, according to officials. Most if not all will be administered to healthcare workers.
Medical authorities in the US and Europe have yet to approve the Pfizer vaccine — or any other of the several in development — which was shown to be 95 percent effective in battling COVID-19.
“The government has today accepted the recommendation from the [Medicines and Healthcare products Regulatory Authority] to approve Pfizer/BioNTech’s Covid-19 vaccine for use,” a UK government spokesperson said, as The Guardian reports.
The news comes shortly after both Pfizer and US-based pharmaceutical Moderna submitted their COVID-19 vaccines to the US Food and Drug Administration for emergency use.
Making the rollout of the Pfizer vaccine challenging is the fact that it needs to be stored at -70 degrees Celsius — far colder than a standard freezer.
“The goal will be to vaccinate through the NHS right across the UK as rapidly as the company can manufacture,” health secretary Matt Hancock told the BBC. “It will help save lives. Once we’ve protected the most vulnerable it will help us all get back to normal and back to some of the things that we love.”
Hancock also noted that after Easter, the country may have a chance of returning to some semblance of normality.