Landmark Discovery
Recently, a bit of stray moisture that made its way into an experiment helped scientists uncover the strange behavior of a complex oxide material that they were studying.
This chance discovery, which was made by several researchers from Drexel University, the University of Pennsylvania, the University of California at Berkely, and Temple University, helped the team better uncover a host a strange properties in the material.
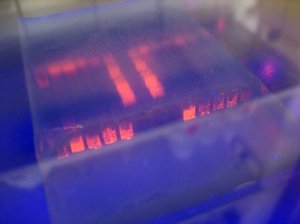
Ultimately, they found that it can emit UV light and has insulating, electrical conducting, superconducting, semiconducting, and ferromagnetic properties—which can (weirdly enough) be controlled by water.
Their discovery was published in the American Chemical Society journal Nano Letters.
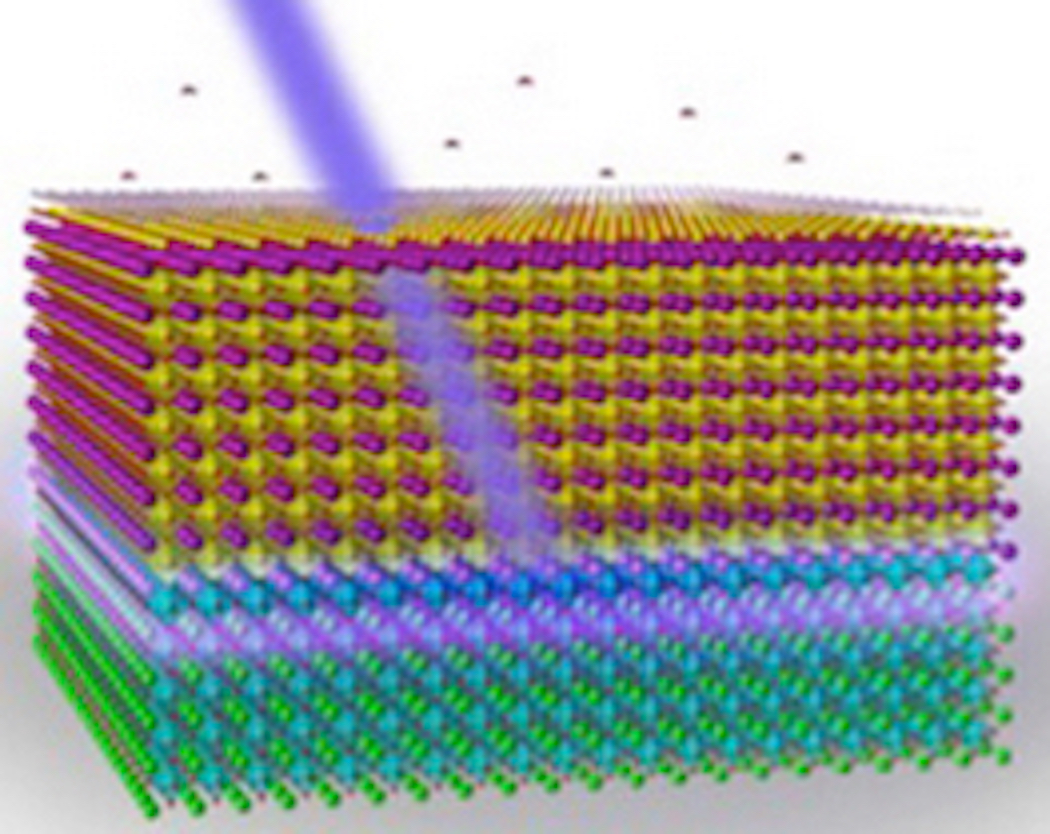
The discovery was made while researchers were studying lanthanum aluminate film on a strontinum titanate crystal. The sample apparently began to glow and emit high levels of UV light. Eventually, the scientist discovered what controlled the UV light to turn on and off: Surface water moisture.
In addition, the researchers also saw that the material exhibited superconducting, ferromagnetic ordering and photoconductive properties.
Just Add Water
“Dissociation of water fragments on the oxide surface releases electrons that move to the buried interface, cancelling out the ionic charges,” Fenggong Wang, one of the theory collaborators on the team said in a release. “This puts all the light emission at the same energy, giving the observed sharp photoluminescence.”
The scientists believe that the material could possibly be used for simple devices, such transistors and chemical sensors.
Of the potential uses, Mohammad Islam, an assistant professor from the State University of New York at Oswego, states, “By strategically placing molecules on the surface, the UV light could be used to relay information — much the way computer memory uses a magnetic field to write and rewrite itself, but with the significant advantage of doing it without an electric current. The strength of the UV field also varies with the proximity of the water molecule; this suggests that the material could also be useful for detecting the presence of chemical agents.”