They said yes
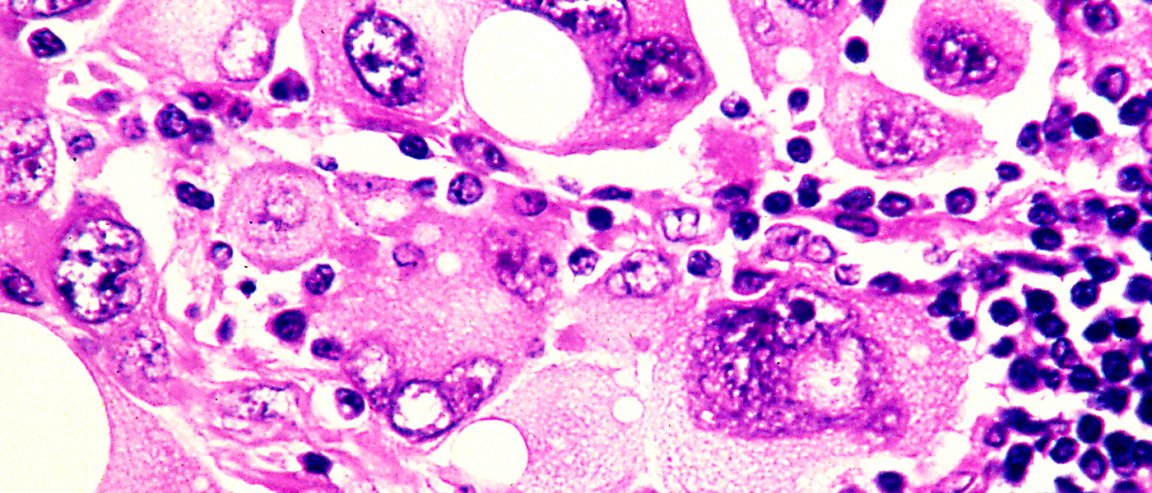
The National Institue for Health and Care Excellence (NICE) recently approved the general use of a combination of two immunotherapy drugs for advanced skin cancer in the National Hearth Service (NHS). This keeps our hopes up that the deadly disease may soon be eradicated or at least stalled.
The use of nivolumab together with ipilimumab was approved by NICE a few months after the combo got its license. According to NICE, patients having advanced melanoma from Wales and England will be the first ones to get it in Europe.
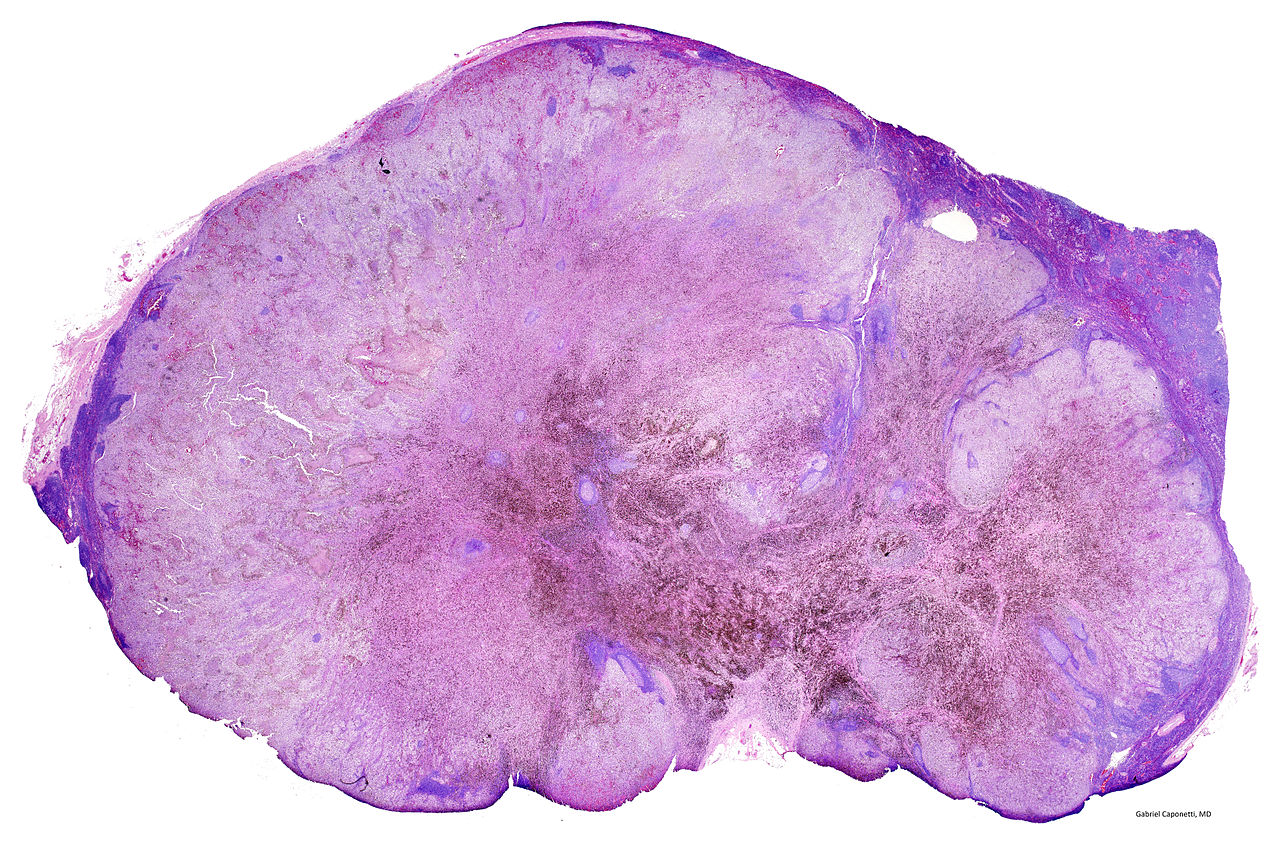
NICE is known to take quite a long time before approving drugs but this time, they surprisingly moved at record speed in processing the approval of the drug. Also, the manufacturer Bristol-Myers Squibb should be thanked for they agreed to offer the drug to NHS at a discounted price, making the process easier.
Patients diagnosed with advanced melanoma are usually given a life expectancy of fewer than two years. Thanks to immunotherapy drugs, those who received the first trials got to live for another ten years or so.
Two is better than one
The drugs teach the immune system to attack cancer cells but when in combination, the drugs sometimes direct the immune system to attack normal cells as well. This could lead to liver damage and other complications. A trial of the combined drug reported last year got a response of 60% far better than previous responses from just ipilimumab alone.
Though NICE’s response to this drug for melanoma is good news, families of cystic fibrosis patients were disappointed when it did not approve the “life-transforming” combination drug Orkambi.
However bittersweet, we have a path forward in the future of medicine.