From Skin to Brain
The brain is one of the most vital organs in the human body, so damage to the brain from injury or aging can have major impacts on people's quality of life. Neurological disorders represent some of today's most devastating medical conditions that are also difficult to treat. Among these is Alzheimer's disease.
Usually, research involving Alzheimer's rely on brain cells from mice. Now, neurobiologists from the University of California, Irvine (UCI) have developed a method that could allow the use of human cells instead of animal ones to help understand neurological diseases better.
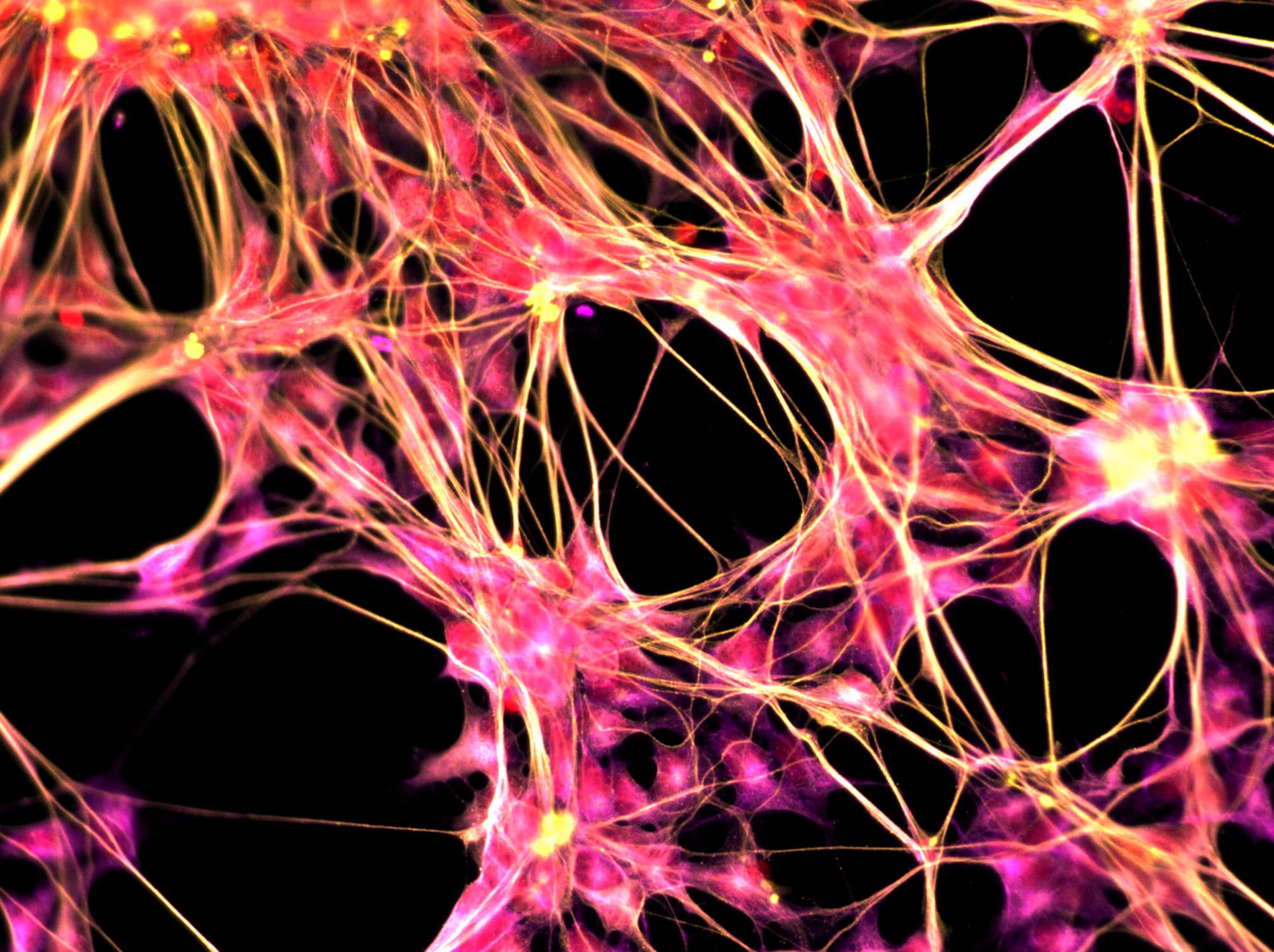
In their study, which was published in the journal Neuron, the researchers found a way to transform human skin cells into stem cells and program them into microglial cells. The latter make up about 10 to 15 percent of the brain and are involved in the removing dead cells and debris, as well as managing inflammation. Micgrolia are instramental in neural network development and maintenance, explained researcher Mathew Blurton Jones, from UCI's Department of Neurobiology & Behavior.
“Microglia play an important role in Alzheimer’s and other diseases of the central nervous system. Recent research has revealed that newly discovered Alzheimer’s-risk genes influence microglia behavior,” Jones said in an interview for a UCI press release. "Using these cells, we can understand the biology of these genes and test potential new therapies.”
A Renewable Method
The skin cells had been donated by patients from UCI's Alzheimer’s Disease Research Center. These were first subjected to a genetic process to convert them into induced pluripotent stem (iPS) cells — adult cells modified to behave as an embryonic stem cell, allowing them to become other kinds of cells. These iPS cells were then exposed to differentiation factors designed to imitate the environment of developing microglia, which transformed them into the brain cells.
“This discovery provides a powerful new approach to better model human disease and develop new therapies,” said UCI MIND associate researcher Wayne Poon in the press release. The researchers, in effect, have developed "a renewable and high-throughput method for understanding the role of inflammation in Alzheimer’s disease using human cells,” according to researcher Edsel Abud in the same source.
In other words, by using human microglia instead of those from mice, the researchers have developed a more accurate tool to study neurological diseases and to develop more targeted treatment approaches. In the case of Alzheimer's, they studied the genetic and physical interactions between the disease's pathology and the induced microglia cells. “These translational studies will better inform disease-modulating therapeutic strategies," Abud added in the press release.
Furthermore, they are now using these induced microglia cells in three-dimensional brain models. The goal is to understand the interaction between microglia and other brain cells, and how these influence the development of Alzheimer's and other neurological diseases.
This is all made possible by reprogrammable stem cells. Indeed, this study is one more example of how stem cells are changing medicine.
Share This Article